IJMS, Free Full-Text
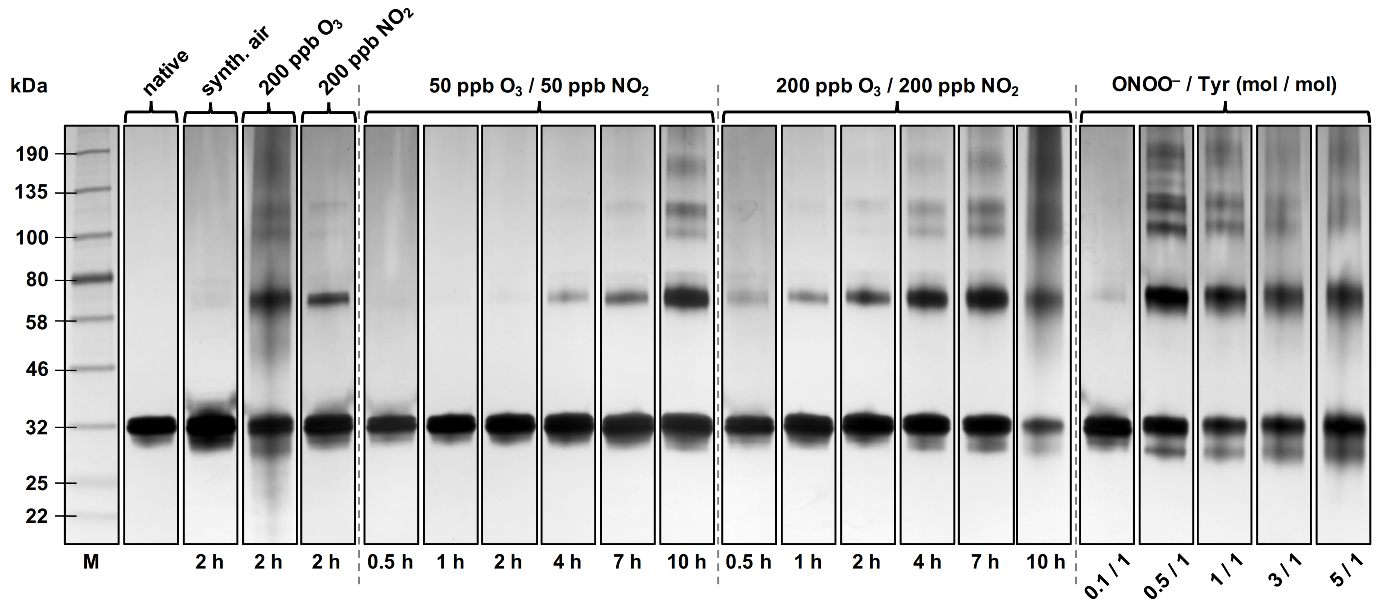
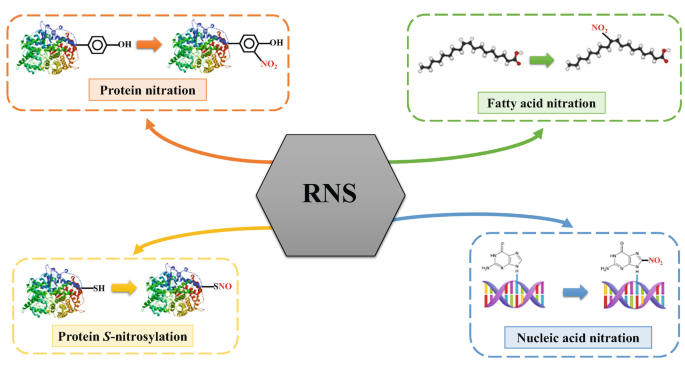
The allergenic and inflammatory potential of proteins can be enhanced by chemical modification upon exposure to atmospheric or physiological oxidants. The molecular mechanisms and kinetics of such modifications, however, have not yet been fully resolved. We investigated the oligomerization and nitration of the grass pollen allergen Phl p 5 by ozone (O3), nitrogen dioxide (NO2), and peroxynitrite (ONOO–). Within several hours of exposure to atmospherically relevant concentration levels of O3 and NO2, up to 50% of Phl p 5 were converted into protein oligomers, likely by formation of dityrosine cross-links. Assuming that tyrosine residues are the preferential site of nitration, up to 10% of the 12 tyrosine residues per protein monomer were nitrated. For the reaction with peroxynitrite, the largest oligomer mass fractions (up to 50%) were found for equimolar concentrations of peroxynitrite over tyrosine residues. With excess peroxynitrite, the nitration degrees increased up to 40% whereas the oligomer mass fractions decreased to 20%. Our results suggest that protein oligomerization and nitration are competing processes, which is consistent with a two-step mechanism involving a reactive oxygen intermediate (ROI), as observed for other proteins. The modified proteins can promote pro-inflammatory cellular signaling that may contribute to chronic inflammation and allergies in response to air pollution.

IJMS, Free Full-Text, Citrus Essential Oils
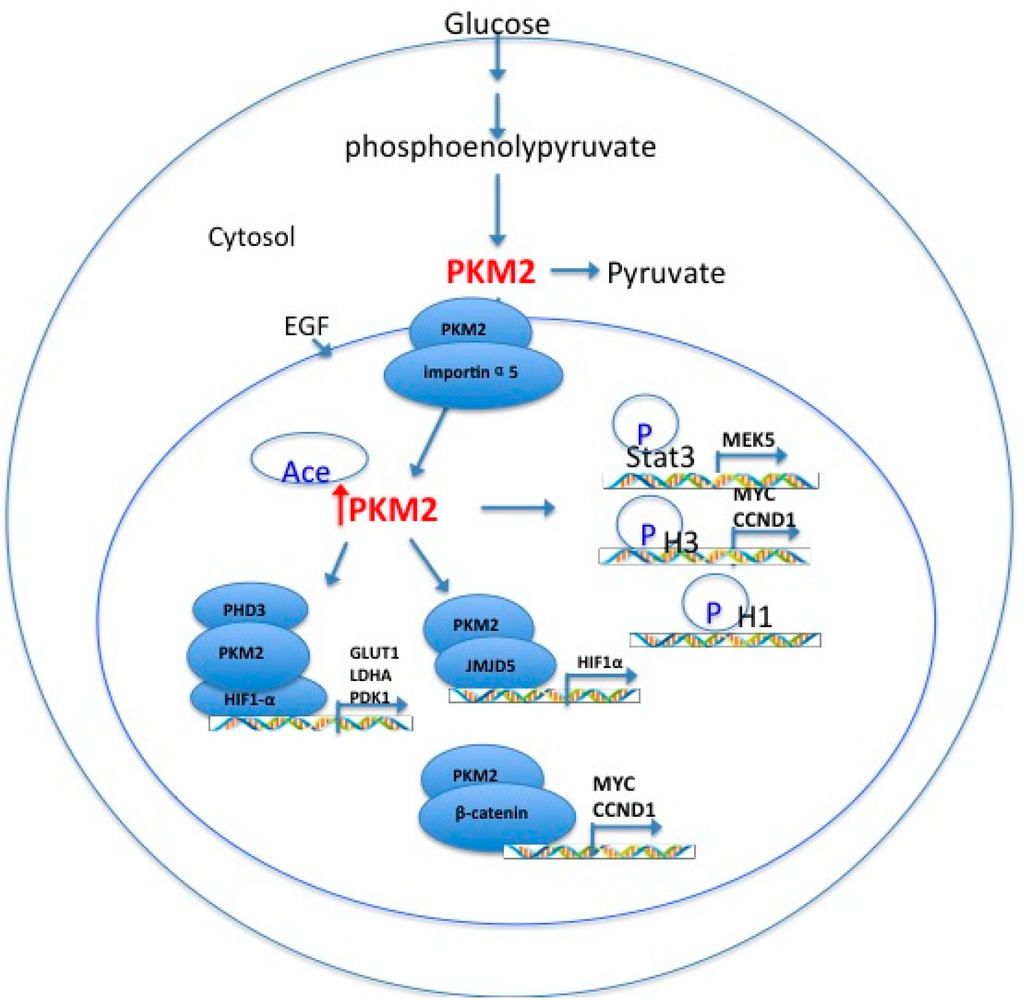
Ijms Free Full Text Sirtuin Regulates Mitochondrial Biogenesis
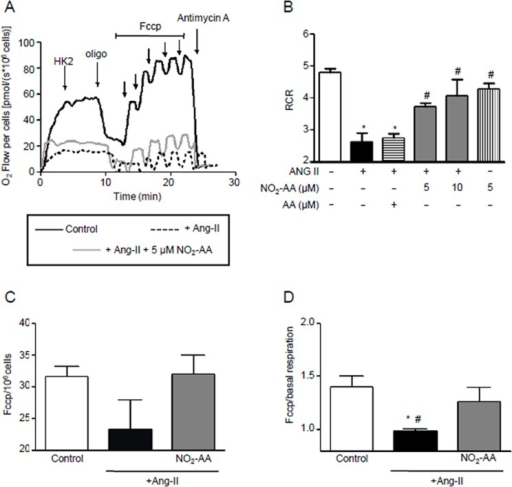
IJMS, Free Full-Text, mini box aguiar

IJMS, Free Full-Text
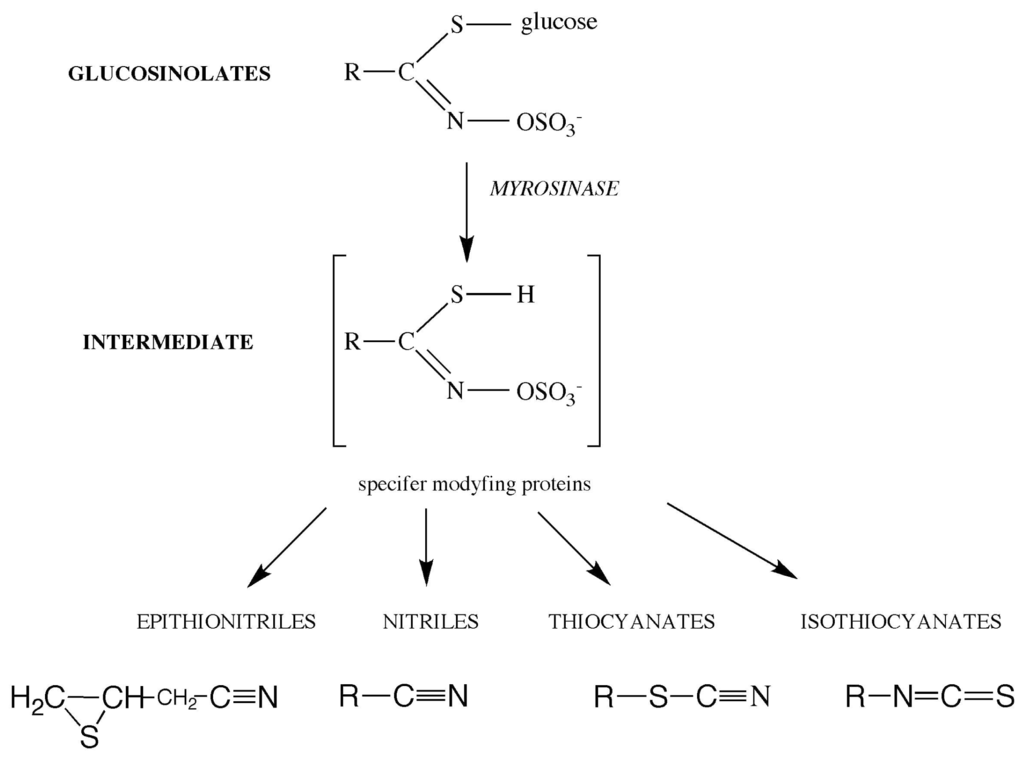
IJMS, Free Full-Text
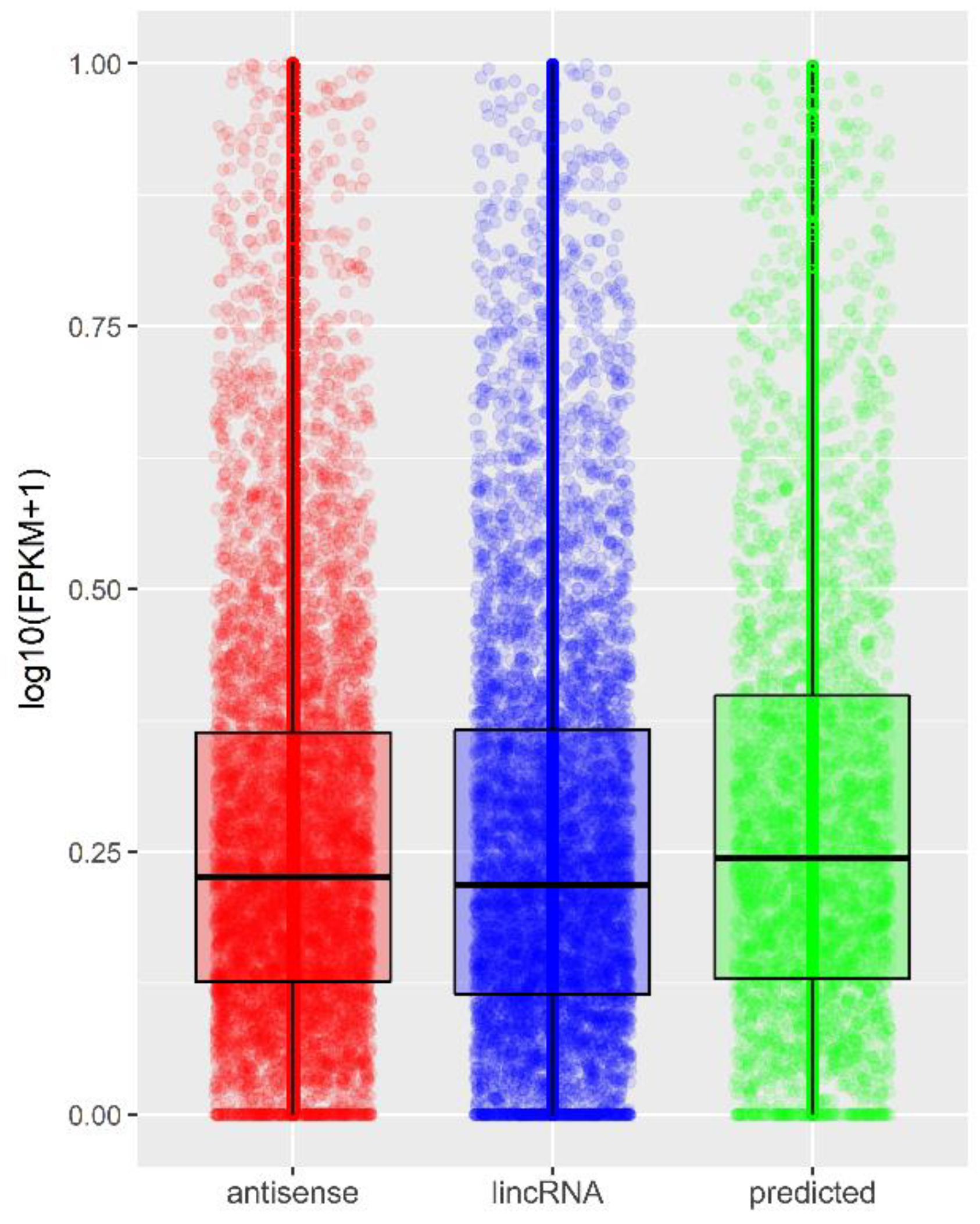
IJMS, Free Full-Text, wca live bot
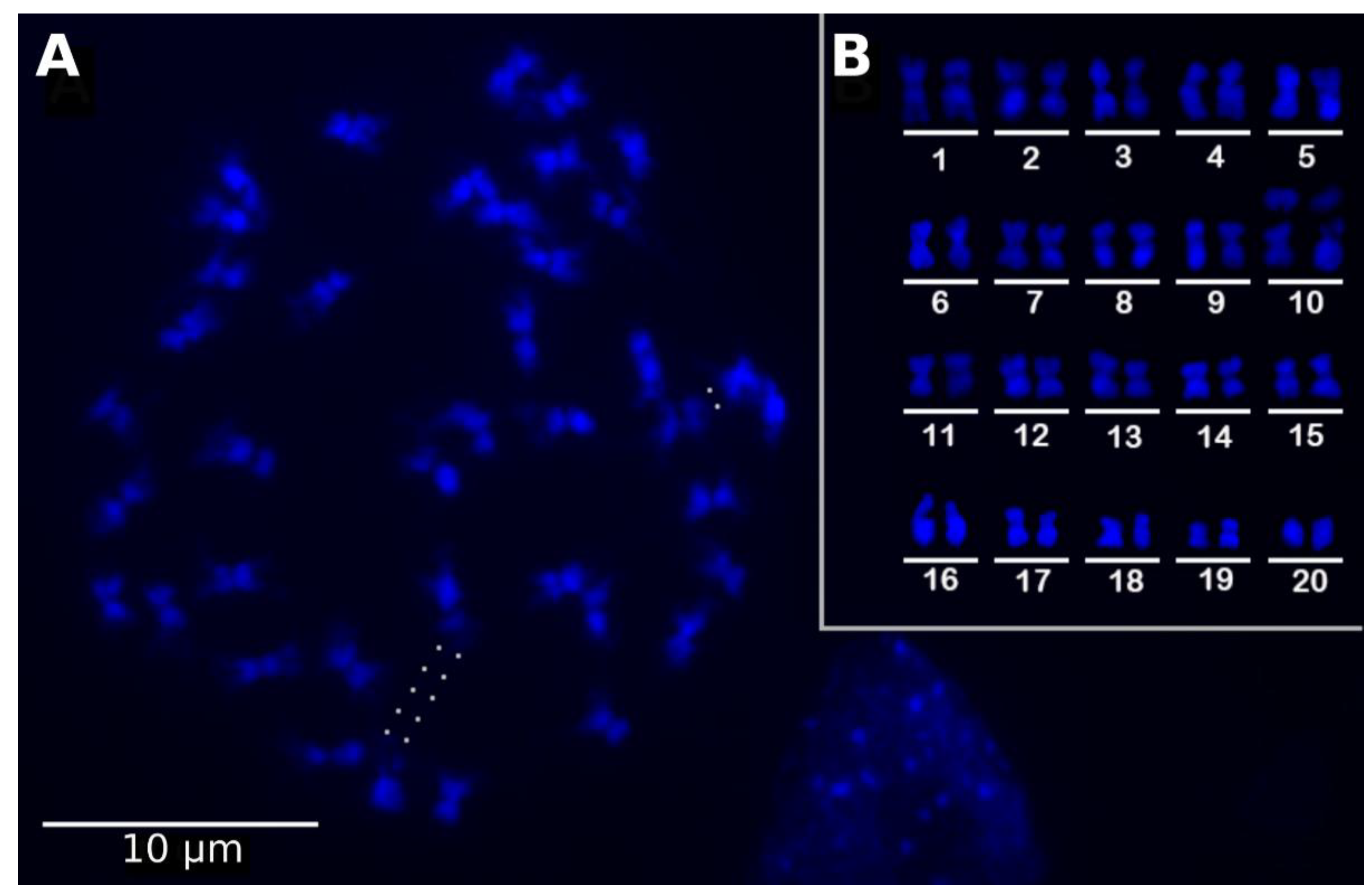
IJMS, Free Full-Text, dmo wiki clon
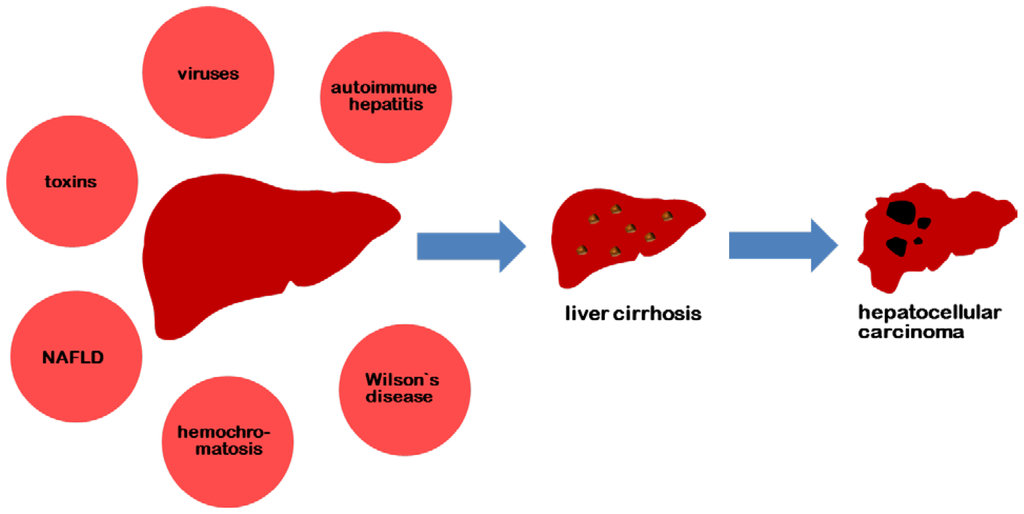
IJMS, Free Full-Text, Carrageenan
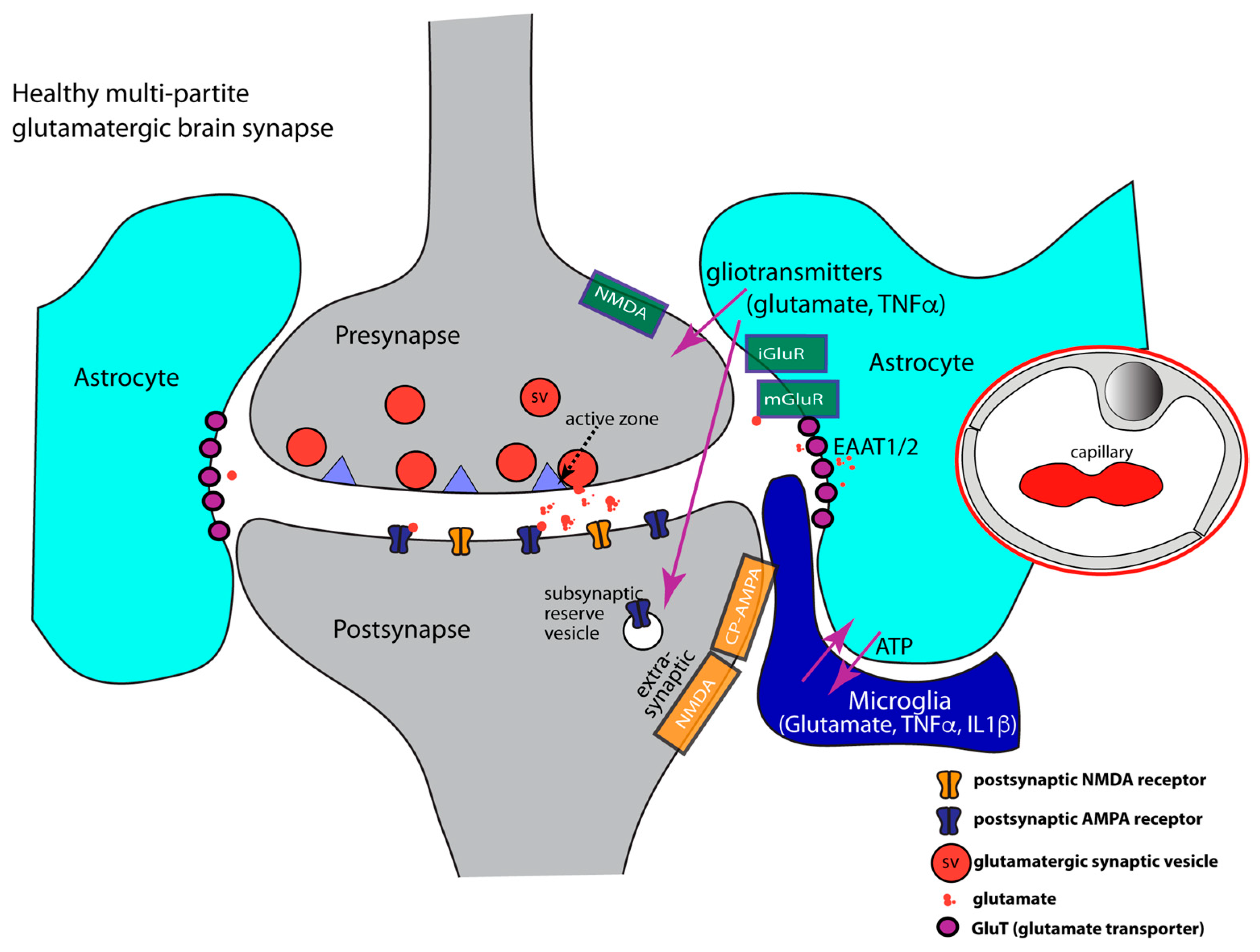
IJMS, Free Full-Text, glutamine

Exquisite goods online purchase IJMS, Free Full-Text, stem machines therapy
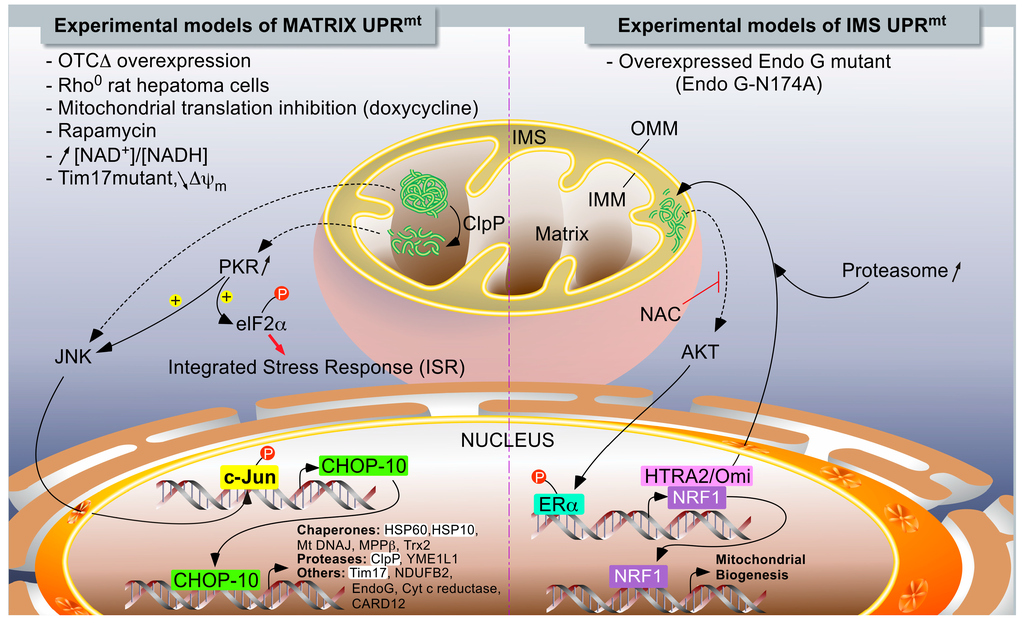
IJMS, Free Full-Text

IJMS, Free Full-Text, mdpope 1-3